MOTS-c Doubles Running Capacity of Old Mice.
“See how they run: ‘Exercise protein’ doubles running capacity, restores function and extends healthy lifespans in older mice
Animal and human data reveal new target for reversing age-related decline, according to USC study.”
“For this study, the research team tested how injections of MOTS-c affected mice of different ages by measuring physical capacity and performance in young (2 months), middle-aged (12 months), and old (22 months) mice. When the mice were presented with physical challenges — including maintaining balance on a rotating rod and running on an accelerating treadmill — mice of all ages who had received MOTS-c treatment fared significantly better than untreated mice of the same age.
Even groups of mice that had been fed a high-fat diet showed marked physical improvement after MOTS-c treatment and less weight gain than untreated mice. These findings echo previous research on MOTS-c treatment in mice, which also found that it reversed diet-induced obesity and diet- and age-dependent insulin resistance.
Additionally, treating the oldest mice nearing the end of their lives with MOTS-c resulted in marked physical improvements. This late-life treatment improved grip strength, gait (measured by stride length) and physical performance, which was assessed with a walking test (running was not possible at this age).
“The older mice were the human equivalent of 65 and above and once treated, they doubled their running capacity on the treadmill,” Lee said. “They were even able to outrun their middle-aged, untreated cohorts.” (1)
MOTS-c Peptide Naturally Found Within Human Tissue During Exercise.
“Humans express MOTS-c with exercise…
To measure the effects of exercise on MOTS-c levels in people, the researchers collected skeletal muscle tissue and plasma from sedentary, healthy young male volunteers who exercised on a stationary bicycle. Samples were collected before, during and after the exercise as well as following a 4-hour rest.
In muscle cells, levels of MOTS-c significantly increased nearly 12-fold after exercise and remained partially elevated after a four-hour rest, while MOTS-c levels in blood plasma also increased by approximately 50% during and after exercise and then returned to baseline after the rest period. The findings suggest that the exercise itself induced the expression of the mitochondrial-encoded regulatory peptides.
The expression of MOTS-c during exercise in humans and the results from the studies in mice lend support to the idea that aging is regulated by genes in both the mitochondrial and nuclear genomes. While further research on MOTS-c is needed, the data indicates that MOTS-c treatment could increase health span, or the portion of the life span spent in good health, and address frailty and other age-related conditions, Lee said.
The results from MOTS-c treatment in mice are extremely promising for future translation into humans, he added, especially the fact that such results were obtained even with treatment starting at older ages.
“Indicators of physical decline in humans, such as reduced stride length or walking capacity, are strongly linked to mortality and morbidity,” he said. “Interventions targeting age-related decline and frailty that are applied later in life would be more translationally feasible compared to lifelong treatments.” (1)

MOTS-c Increases Creation of Bone.
“MOTS-c accelerates bone fracture healing by stimulating osteogenesis of bone marrow mesenchymal stem cells via positively regulating FOXF1 to activate the TGF-β pathway…”
“MOTS-c treatment upregulated the relative levels of ALP, Bglap, and Runx2, and stimulated mineralization ability in BMSCs, which were attenuated by the silence of FOXF1. TGF-β was proved to interact with FOXF1, and its level was positively mediated by FOXF1. The silence of FOXF1 attenuated the accelerated osteogenesis and TGF-β upregulation in BMSCs because of MOTS-c induction, and these trends were further reversed by the overexpression of TGF-β.
*MOTS-c treatment markedly induces osteogenesis in BMSCs [bone marrow mesenchymal stem]. During MOTS-c-induced osteogenic progression, the upregulated FOXF1 triggers the activation of TGF-β pathway, thus accelerating bone fracture healing.” (2)

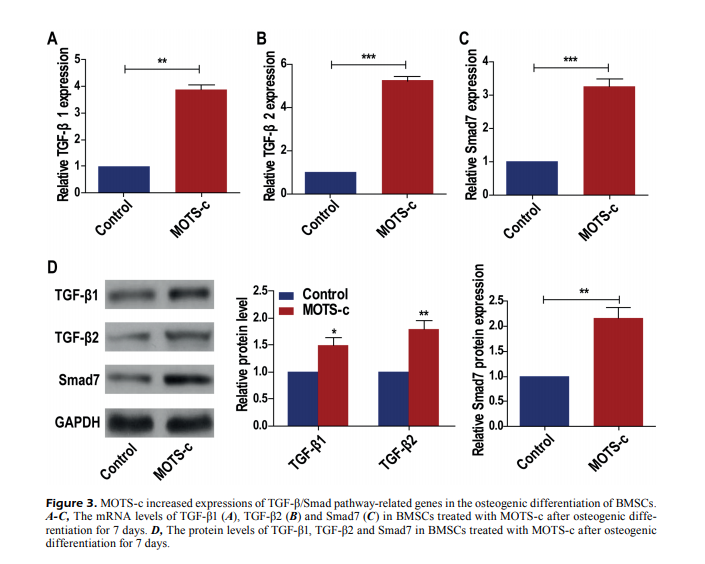
“MOTS-c improves osteoporosis by promoting osteogenic differentiation of bone marrow mesenchymal stem cells via TGF-β/Smad pathway…”
“MOTS-c treatment could remarkably stimulate the formation of calcified nodules in BMSCs. Besides, TGF-β/Smad pathway-related genes were significantly upregulated after BMSCs were treated with MOTS-c. Promoted osteogenesis by MOTS-c treatment was reversed by the TGF-β1 knockdown. MOTS-c promotes cell differentiation of BMSCs to osteoblasts via TGF-β/Smad pathway.” (3)
MOTS-c Released by Mitochondria During Systemic Responses.
“…in the nuclear and mitochondrial DNA there are places in which it is possible to codify peptides of less than 100 codons called open reading frames (ORF) potentially able to be translated into peptides and small proteins. A lot from these peptides in eukaryotic cell have its origin from larger proteins and suffer some kind of processing, but in the human genome there are hundreds to thousands of places capable to transcript these small peptides.
It is important to stress that as well as the nuclear genome, the mitochondrial genome also have these structural open reading spaces, being identified transcripts of protein of systemic action, known as mitochondrial-derived peptides (MDPs), among others, Humanin & MOTS-c as the most notorious of them.
This peptide performs various functions, as an important cytoprotector in helping to maintain the mitochondrial function and the cellular viability under stressful conditions, regulating the metabolic homeostasis. The treatment in mice with MOTS-c showed itself as capable to avoid age resistance to insulin and also obesity induced by a diet rich in fat…
…this interesting organelle [mitochondria] is considered an important local generator of systemic responses. New evidence has pointed mitochondria as a local of larger genic repertoire. These findings involve the discovery of two peptides named Humanin and mitochondrial open Reading frame (ORF) of the twelve S-c (MOTS-c), which are derivative from mtDNA and have an important systemic performance, breaking paradigms in relation to the physical intracellular space of mitochondrial operation.
With its importance, it is not surprising to know that mitochondria also are sensitive to diverse intrinsic stressor agents such as the mutation and deletion of the nuclear desoxyribonucleic acid (DNA) or mtDNA, privation or excess of energetic substrates, increase of ROS and also to stressor extrinsic agents, such as toxins, viruses, bacteria and ultraviolet rays. These agents can alter both mitochondrial function and dynamic that integrate many pathways aiming the necessary energy supply so that the cell is able to adapt to these stressor agents.” (4)
MOTS-c Boosts Fat Oxidation, Catabolism, and Heat/Energy Production.
“Since MOTS-c inhibits indirectly the biosynthesis of purines, there is an elevation of AICAR (5-aminoimidazole-4-carboxamide ribonucleotide), an intermediate of purine synthesis. This increase in AICAR activates the kinase activated by 5′ adenosine monophosphate-activated protein kinase (AMPK), which enhances the fat oxidation [38]. The AMPK also inhibits the Aceti-CoA – Carboxilase, which its function is transforming Acetil-Coa in Malonil-Coa – and this last one performs an allosteric inhibitory effect in the Palmitoil-Transferase 1. So, when AICAR inhibits Acetil-CoA-Carboxilase, it allows that more fatty acids enter in the mitochondria and be available for the β-oxidation. These catabolic effects combined increase the lipolysis, reduce the lipogenesis and increase the reception of glucose.
Even more interesting, AMPK activates the coactivator alpha 1 of the Peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α), which is a coactivator of genic transcription. Between the nuclear transcription targets of PGC-1α, there is the Nuclear respiratory factor (NRF-1 e NRF-2) and members of the Peroxisome proliferator-activated receptor (PPAR), all of them involved with the increase of proteins that regulate or are part of the mitochondrial structure. One of the NFR-1 products is the Mitochondrial transcription factor A (TFAM) that goes to the mitochondria increasing the transcription of mitochondrial gens.
Furthermore, rodents treated with injections of MOTS-c presented an increase of heat production and energetic consumption. This is analogue to the increase of the activity of uncoupler proteins (UCPs), which are activated by the stimulation adrenergic of the sympathetic nerves that innervate the adipose brown tissue an increase the activity of the second messenger cyclic adenosine 3′, 5′-monophosphate (cAMP). This activates the AMPK that accelerates catabolic pathways with the lipolysis and increases the transcription of UCP-1. This adaptive mechanism refers to the thermogenic process of dissipation of heat and increase of body temperature, resultant of the adenosine triphosphate (ATP) synthesis decoupling through the leak of protons from the internal mitochondrial membrane, known as thermogenesis without tremor, which can explain the increase of the energetic consumption and production of heat. If MOTS-c is able to increase the activity and expression of UCPs it is a scenario to be further investigated.”
Triple Targeting of Catabolic Pathways While Preserving Bone Strength And Glucose Clearance.
“For example, our research group has already showed that the ovariectomy promotes a case of dyslipidemia and increase in body mass, both related to the risk of increase of metabolic syndrome. In all these works, a RT protocol was able to reduce said risks. In similar fashion, a work of Lu et al. has showed that rats that underwent an ovariectomy and were treated with MOTS-c had prevention in terms of increasing body mass in the group. Besides, the OVX-MOTS-c group showed higher activity of the brown adiposus tissue, evaluated by means of mitochondrial markers how the increase of the expression PGC-1α, UCP-1, number and quantities of mitochondrial crista. It is also interesting that our groups have also showed an increase of PGC-1α expression in the OVX group trained in the skeletal muscle tissue, like what happened in administration with MOTS, however evaluated in different tissues.
Another RT effect known is to prevent and treat cases of osteoporosis and osteopenia common in low levels of estrogen, both in the animal model of ovariectomy as in studies with humans. In this context, MOTS-c also mimicked the effects of physical exercise, being able to prevent significantly the loss of bone mass induce by ovariectomy analyzed by means of micro-CT. The role of MOTS-c as a prevention factor for bone loss in this study is related trough the inhibition of osteoclast formation induced by receptor activator of nuclear factor kappa-Β ligand (RANKL) via activation of AMPK. Between the physiological effects that MOTS-c promote and that emulate physical exercise effects, it is worth to mention the inhibition of the increase differentiation of osteoclasts and reduction of proinflammatory cytokines in animal models.
Another pathway that must be investigated regarding the operation of MOTS-c is its ability of increasing the quantity of AICAR (5-aminoimidazole-4-carboxamide ribonucleotide). As seen in the subitem MOTS-c biological effects, in the study led by Lee et al., the metabolites in cells in culture showed reduction in the levels of active folate, 5Me-THF, molecule that is enzymatic coactivator of nucleotides biosynthesis. The reduction of 5Me-THF increased levels of AICAR, intermediate of the synthesis “de novo” nucleotides, that also has many metabolic effects.
AICAR is an agonist of AMPK, well studied in animal models. AMPK activity increases when has decreased ATP levels occurs. Between targets AMPK we have PPAR-α, and PPAR-Δ, PGC-1α, which are pathways that lead to the increase of the energetic contribution of catabolic pathways, similar to what happens in the physical exercise. Equivalent to the adaptation process that happen in the physical exercise, administration of AICAR enhanced in 44% the performance of mice running in the treadmill then control group.
The physical exercise canalizes to similar pathways such as the ones described above because it increases the energetic demand required to the achievement of physical exercise, elevating the levels of the relation adenosine monophosphate (AMP)/ATP that activate AMPK, PGC-1α, and PPAR-γ.
These described pathways could be the link between the new group of MOTS-c and mitochondria adaptations that aim to provide energetic supply to the cell, as well as converging to important metabolic adaptations. MOTS-c better the glucose tolerance in animals and physical exercise in human too. MOTS-c better metabolic conditions in diets rich in fat as well as in physical exercise, both in animal models. Besides, MOTS-c increases cellular levels of AICAR the same way the physical exercise increases AMPK levels, which is activated by AICAR. So, it is plausible that interconnected pathways happens in the administration of MOTS-c and also in the physical exercise. However, if MOTS-c is a signal of physical exercise it is an issue that must be verified in future studies.” (4)
Sourced Studies:
Product available for research use only:

