Novel Peptides Show Potential to Strengthen Bone Density in Osteoporosis Patients
Osteoporosis is the most prevalent systemic skeletal system disease, leading to increased bone fragility and vulnerability to fractures. Due to the microarchitectural destruction in bone tissue, fracture healing in osteoporotic patients is often delayed and compromised compared with non‑osteoporotic individuals. Osteoporosis usually results from menopause, aging, metabolic diseases and drug therapies with the precise cellular and molecular mechanism remaining to be elucidated.
Recent studies have shown that four peptides (BPC-157, AOD 9604, MOTS-c, Peptide 11R‐VIVIT) have been proven to have healing effects for such disease in several types of models.
BPC-157 Introduction
BPC-157 is a synthetic amino acid sequence of the naturally occurring Body Protection Complex (BPC) found in human gastric juice. BPC-157 has been shown to improve healing of many types of wounds, accelerate healing of skin burns, work as an anti-inflammatory, protect and promote healing of the liver due to toxic stress, improve digestive function, protect intestinal organs, prevent stomach ulcers, and repair tissues of GIT, tendons, ligaments, the brain, bones, and more.
BPC-157 and Osteoporosis
Gastrectomy, surgical removal of a part or the whole of the stomach, often results in increased likelihood of osteoporosis, metabolic aberration, and risk of fracture, and there is a need for a gastric peptide with osteogenic activity. BPC-157, a novel pentadecapeptide, has shown to improve fracture and wound healing in rats as well as having an angiogenic effect.
Using a segmental osteoperiosteal bone defect that remained incompletely healed in all control rabbits for 6 weeks, researchers were able to study the effects of BPC-157 percutaneously given locally into the bone defect, intramuscularly, and continuously. The rabbits were assessed at 2 week intervals. Rabbits percutaneously received locally autologous bone marrow for comparison. As standard treatment, immediately after its formation, the bone defect was filled with an autologous cortical graft. Saline-treated injured animals were used as controls.
“Pentadecapeptide BPC-157 significantly improved the healing of segmental bone defects. For instance, upon radiographic assessment, the callus surface, microphoto densitometry, quantitative histomorphometry (10 mg/kg body weight i.m. for 14 days), or quantitative histomorphometry (10 ng/kg body weight i.m. for 14 days) the effect of pentadecapeptide BPC-157 was shown to correspond to improvement after local application of bone marrow or autologous cortical graft.”
In summary, IM administration of BPC-157 produced results comparable with percutaneous injection of autologous bone marrow, or autologous bone grafting (the effectiveness could be seen also after local administration). In the light of the suggested stomach significance for bone homeostasis, the possible relevance of BPC-157’s effect (local or intramuscular effectiveness, lack of unwanted effects) could be a basis for methods of choice in the future management of healing impairment in humans, and requires further investigation. Finally, peptide in toxicological studies is apparently nontoxic, even applied in very high doses, and it could be a promising basis for further management of healing impairment in patients.
AOD-9604 Introduction
AOD-9604 is a peptide that constitutes a fragment of GH without the proliferative effects which has several effects on bone. It is an 8% fragment of the GH molecule which activates both the IGF-1 and direct cellular pathways. AOD-9604 does not act on the IGF-1 pathway which causes significant insulin resistance. This product has been used with increasing success in treating local pain: eg, tendonitis, osteoarthritis.
AOD-9604 and Osteoporosis
Researchers out of South Korea investigated the effects of AOD-9604 intra-articular injections with or without hyaluronic acid (HA) in a collagenase-induced knee osteoarthritis (OA) rabbit model.
Thirty-two mature New Zealand white rabbits were randomly administered 2mg collagenase type II twice in each knee joint. Group 1 was given weekly injections of 0.6 mL saline, group 2 was given 6mg HA, group 3 was administered 0.25mg AOD 9604, and group 4 was given 0.25mg AOD 9604 with 6 mg HA. After the first intra-articular collagenase injection, each injection was administered for 4-7 weeks. Using morphological and histopathological findings, the degree of cartilage degeneration was assessed. The degree of lameness was also observed at 8 weeks after the first collagenase injection.
Gross morphological and histopathological findings according to four different injections is shown in Figure 1. Using this method to compare results, scores were significantly higher in Group 1 than in Groups 2, 3, and 4, and the scores were significantly lower in Group 4 than in Groups 2 and 3. The lameness period in Group 4 was significantly shorter than those in Groups 1, 2, and 3. The lameness period in Group 1 was significantly longer than those in Groups 2 and 3.
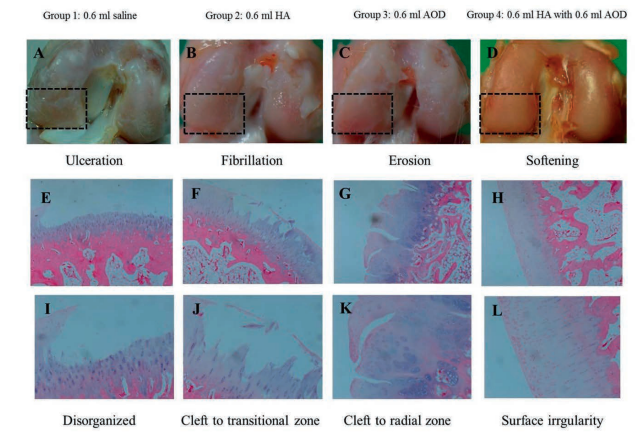
Figure 1. Gross morphological (rectangular areas) and histological (hematoxylin and eosin staining, ×40 and ×100) findings of knee osteoarthritis in four groups. Group 1: normal saline (A,E,I), group 2: hyaluronic acid (B,F,J), group 3: AOD9604 (C,G,K), Group 4: AOD9604 with hyaluronic acid (D,H,L).
All in all, “intra-articular AOD9604 injections using ultrasound guidance enhanced cartilage regeneration, and combined AOD9604 and HA injections were more effective than HA or AOD9604 injection alone in the collagenase-induced knee OA rabbit model”.
MOTS-c Introduction
Human mitochondrial DNA (mtDNA) encodes 37 known genes, including 2rRNAs, 22 tRNAs and 13 polypeptide subunits of the electron transport chain (ETC) complexes. Recent work has revealed that the rRNA loci contain small open reading frames (ORFs) that can be transcribed and translated into short peptides called mitochondrial-derived peptides (MDPs), which have biological activity. MOTS-c is a mitochondrial-encoded peptide with 16-aa’s encoded within the 12S rRNA locus of mtDNA in human cells.
MOTS-c can translocate into the nucleus in response to metabolic stress and regulation of adaptive nuclear gene expression. This allows the peptide to promote resistance of metabolic stress by upregulating the mitochondrial genome. Upregulating these genes encourages mitochondrial biogenesis. MOTS-c inhibits the methionine-folate cycle resulting in purine synthesis, increase in PCG-1α (a key regulator of energy metabolism), and AICAR (5-Aminoimidazole-4-carboxamide ribonucleotide) accumulation which activates AMPK (5’- adenosine monophosphate-activated protein kinase). This acts as an energy sensor by monitoring the ratio of AMP and ATP. AMPK restores homeostasis by initiating catabolic processes for ATP production in case of energy deficits. In addition, literature suggests that MOTS-c decreases insulin resistance and increases GLUT4 uptake in muscle. The peptide is mainly used for weight loss (regulating muscle and fat metabolism) and energy (cell survival in toxic conditions). MOTS-c is consistently used by sports performance athletes to enhance one’s performance. It also displays a promising effect on longevity. The Japanese long-lived people (population with the longest lifespan in the world) have demonstrated a phenotypic expression and biological link between MOTS-c and an extended lifespan.
MOTS-c and Osteoporosis
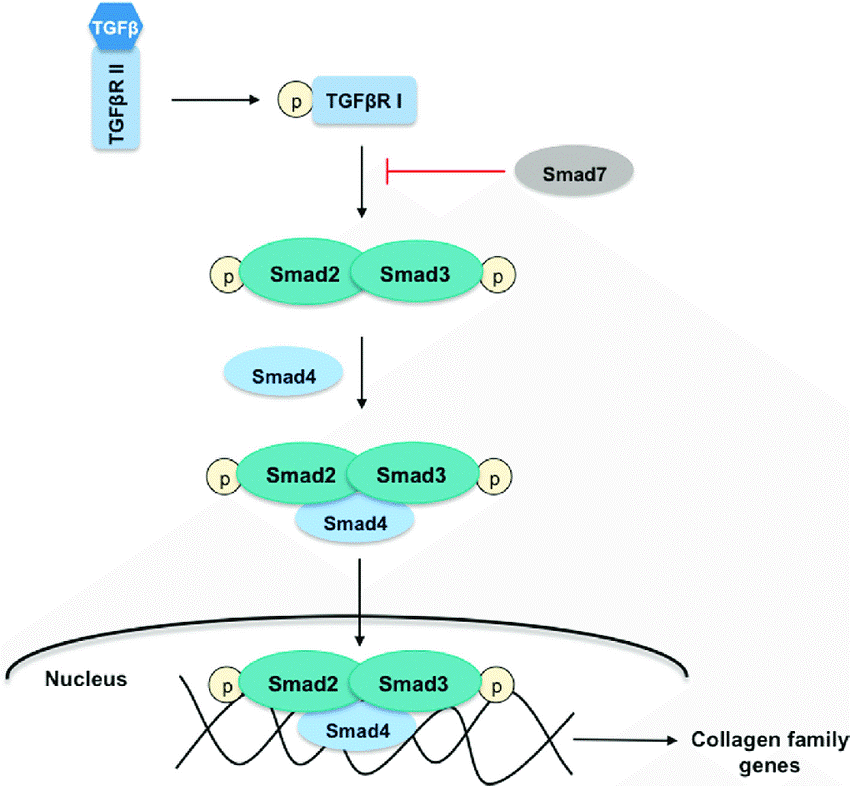
Few studies have reported the effect of TGF-β/SMAD signaling pathway (seen below) on stimulating osteoblasts to secrete type I collagen. This study below aimed to investigate the role of MOTS-c in the synthesis of type I collagen in osteoblasts, which provides new suggestions for discovering the pathogenesis and treatment of osteoporosis.
“The viability of hFOB1.19 cells (a cell line cultured in medium containing 10% fetal bovine serum and 100 U/ml penicillin and 100 mg/mL streptomycin) were treated with MOTS-c and were detected by a CCK-8 assay. The mRNA and protein levels of TGF-β, SMAD7, COL1A1 and COL1A2 in hFOB1.19 cells were detected by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot, respectively. Researchers then changed expressions of TGF-β and SMAD7 by plasmid transfection to detect levels of COL1A1 and COL1A2 in hFOB1.19 cells by qRT-PCR and Western blot.”
Cell viability was significantly increased after treatment of 1.0 μM MOTS-c for 24 h or 0.5 μM MOTS-c for 48 h in a time-dependent manner. The mRNA and protein expressions of TGF-β, SMAD7, COL1A1 and COL1A2 in hFOB1.19 cells were dependent on the concentration of MOTS-c. In addition, MOTS-c increased the expressions of COL1A1 and COL1A2, which were partially reversed by knockdown of TGF-β or SMAD7. See Figure 1 below.

Figure 1. MOTS-c contributed to the synthesis of type I collagen in hFOB1.19 cells via TGF-β/SMAD pathway. A-B, The hFOB1.19 cells were divided into the control group, MOTS-c group and MOTS-c TGF-β inhibitor group. The expressions of COL1A1 and COL1A2 were detected by qRT-PCR. C-D, The hFOB1.19 cells were divided into the control group, MOTS-c group and MOTS-c SMAD7 inhibitor group. The expressions of COL1A1 and COL1A2 were detected by qRT-PCR.
High concentration and long-term stimulation of TGF-β1 induced osteogenic differentiation of bone marrow mesenchymal stem cells (MSCs) in vitr2. TGF-β pathway-related genes exert anti-osteoporosis effects by regulating the function of bone deposits and osteoclasts. TGF-β also affects the bone formation by promoting the proliferation and differentiation of osteoblasts, as well as the synthesis of extracellular matrix. In conclusion, MOTS-c can promote osteoblasts to synthesize type I collagen via TGF-β/SMAD pathway.
Peptide 11R‐VIVIT Introduction
Peptide 11R‑VIVIT is a NFAT‑specific inhibitor without affecting upstream calcineurin (Cn). Very few adverse effects have been reported on peptide 11R‑VIVIT due to its high specificity against NFAT2, and being widely examined. “The nuclear factor of activated T‑cells (NFAT) is a substrate of the Ca2 ‑dependent transcription factor family, which has been shown to regulate cell differentiation and organ development, podocyte injury and cell apoptosis.
In addition, NFAT signaling has been found to play critical roles in bone metabolism, such as in the process of osteoblast and osteoclast formation. In detail, the activation of the NFAT signaling pathway has been shown to lead to the inhibition of osteoprogenitor cell formation.” By contrast, the blocking of NFAT signaling contributes to the osteoblastic differentiation of mesenchymal stem cells (MSCs), which suggests that NFAT is a potential target for the treatment of osteoporotic fractures.
Peptide 11R‐VIVIT and Osteoporosis
A previous study demonstrated that 11R‑VIVIT can stimulate bone formation by decreasing NFATc1 expression and regulating the expression of inflammation‑related molecules. However, whether peptide 11R‑VIVIT can affect the healing process of osteoporotic fractures via the downregulation of NFATc1 remained unknown before the study described below.
In March of 2021, researchers aimed to investigate the repair properties of 11R‑VIVIT on osteoporotic fractures and to examine the potential effects of 11R‑VIVIT on osteoporotic bone marrow‑derived mesenchymal stem cells (BMSCs).
“A rat model of osteoporotic femoral fracture was established, and the effects of the daily local injection of 11R‑VIVIT or saline on fracture repairing were evaluated by micro‑CT scans and H&E staining. Moreover, BMSCs from osteoporotic rats were treated with 11R‑VIVIT, and the osteogenic and adipogenic differentiation of BMSCs was evaluated.”
In conclusion, the present findings demonstrated that 11R‑VIVIT enhanced rat osteoporotic fracture healing by modifying AKT/NFATc1 signaling. Moreover, 11R‑VIVIT may also be responsible for promoting the shift of osteoporotic BMSCs to an osteoblastic phenotype, and may promote bone formation during osteoporosis.
The results indicated that 11R‑VIVIT promoted autophagy to enhance the osteogenic differentiation of osteoporotic BMSCs to promote fracture healing in osteoporotic rats through the protein kinase B (AKT)/NFATc1 signaling pathway, which may provide new insight for the BMSC‑based treatment of osteoporosis in the future.
References:
Products available for research use only: