Causes of Alzheimer’s… Synapse degeneration, Beta amyloid plaques, and Tau protein build-up.
“Currently 25–35 million people worldwide suffer from AD, the single largest cause of dementia in middle- to old-aged individuals.” (1) “Approximately 476,000 people age 65 or older are expected to develop AD in the United States in the year 2016, and the total healthcare expenditure for AD and related dementias will be nearly 236 billion dollars, making it one of the costliest chronic diseases in the United States. Worldwide, AD and related dementias affect approximately 47 million people. As yet there is no effective cure for AD. By the year 2050, the prevalence of AD is projected to be 13.8 million in United States, and 135 million worldwide, lest an effective AD therapy is developed.” (4)

“Synaptic loss is a better correlate of cognitive impairment in AD than Aβ or tau pathologies. Thus a highly promising therapeutic strategy for AD is to shift the balance from neurodegeneration to neuroregeneration and synaptic repair. Neurotrophic factors, by virtue of their neurogenic and neurotrophic activities, have potential for the treatment of AD.” (4)
“AD has been described as a synaptic failure. AD brains also show dendritic and dendritic spine loss. Quantitative studies on AD brains within 2–4 years after the clinically diagnosed disease showed a 25–35 % decrease in synaptic density and a 15–35 % synaptic loss per neuron in the frontal and temporal lobes of the cerebral cortex. The extent of synaptic loss is even more severe in the hippocampus where it amounts to 44–55 %. Remarkably, the synaptic loss in frontal cortex and limbic system is the best correlate of the severity of cognitive dysfunction. The profound neuronal, synaptic, and dendritic loss may contribute to impaired synaptic plasticity, including reduced LTP, in AD.” (4)
“The early-onset familial form of AD which is caused by mutations in amyloid β precursor protein (APP), presenilin 1 (PS1), or presenilin 2 (PS2), accounts for <1 % of all cases… The exact causes of the late-onset sporadic form of AD, which accounts for over 99 % of the cases, are not yet understood.” (4)

“The histopathological hallmarks in both the sporadic and the familial forms of AD are similar and include amyloid β plaques and NFTs leading to neurodegeneration.
• “Tau which is a neuronal microtubule associated protein (MAP) is a major protein subunit of paired helical filaments that constitute the NFTs. Tau in NFTs is abnormally hyperphosphorylated. Tau is a substrate for several protein kinases including cyclin-dependent kinase 5 (Cdk5), glycogen synthase kinase 3 β (GSK-3β), and calcium/calmodulin activated protein kinase II (CaMKII). The activities of several major tau kinases are regulated by protein phosphatase 2A (PP2A). PP2A can thus regulate the tau phosphorylaiton both directly and by inhibiting the activities of tau protein kinases. PP2A activity is impaired and is possibly a cause of the abnormal hyperphosphorylation of tau in AD brain.” (4)
• “The immensely popular amyloid cascade hypothesis suggests a major etiological role of Aβ for the NFT pathology, neurodegeneration and cognitive dysfunction in AD. Aβ plaques are produced by amyloidogenic processing of the transmembrane protein, APP, by β- and γ-secretase enzymes resulting in the formation of major toxic products, Aβ40 and Aβ42 peptides. The oligomeric form of Aβ has also been suggested as the main neurotoxic state of the peptide. Aβ oligomers are known to inhibit hippocampal long-term potentiation (LTP), a major cellular mechanism underlying synaptic plasticity, and learning and memory. Amyloid cascade hypothesis also derives its major support from the Down syndrome individuals; as part of the trisomy 21, these patients carry 3 copies of APP and almost all of them develop AD neuropathological characteristics by the age of 40 years.” (4)
“The two histopathological hallmarks of AD and adults with Down syndrome, who develop AD histopathology in the fourth decade of their lives, are the intraneuronal neurofibrillary tangles and the extracellular Aβ plaques. The tangles in AD as well as in related neurodegenerative diseases called tauopathies are made up of microtubule associated protein tau modified by its abnormal hyperphosphorylation. The major components of plaques are 39–43 amino acid fragments, mostly Aβ1–40 and Aβ1–42 of β-amyloid precursor protein. Ever since the discoveries of the composition of Aβ plaques and neurofibrillary tangles and implication of those lesions in neurodegeneration, most of the research in the AD field has been focused on inhibition of neurodegeneration by prevention or clearance of these lesions.” (1)

“Histopathologically, there are two major lesions in AD: senile plaques (diffuse and neuritic) and neurofibrillary tangles (NFTs). Amyloid β (Aβ) is the main component of the senile plaques whereas abnormally hyperphosphorylated tau protein gives rise to NFTs. Alongside Aβ plaques and NFTs, impairments in adult hippocampal neurogenesis and synaptic plasticity, synaptic deficit, and profound neurodegeneration are also major features of AD. Adult hippocampal neurogenesis and synaptic plasticity have been proposed to be essential for learning and memory. Alterations in adult hippocampal neurogenesis and synaptic plasticity could thus be the major mechanisms underlying cognitive dysfunction in AD. AD has also been characterized as a consequence of synaptic failure. Several studies have shown that synaptic loss correlates better with cognitive decline than either Aβ plaque load or NFTs.” (4)
In Alzheimer’s disease, degeneration of brain synapses happens before Beta Amyloid plaques and Tau protein aggregates are produced.
“Both in AD and in its animal models the loss of neuronal plasticity is known to precede any overt formation of Aβ plaques and hyperphosphorylated (p) tau neurofibrillary tangles.” (1)
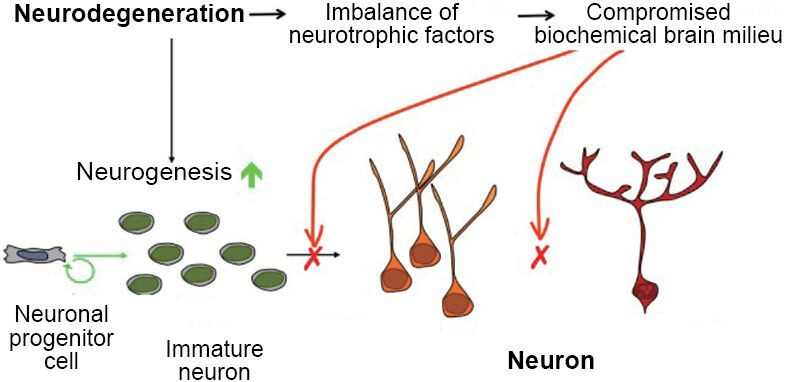
“Alzheimer’s disease responds to neurodegeneration by initiating neurogenesis in the dentate gyrus which, however, due to a lack of the proper neurotrophic support, is not sustained and the newborn neurons do not mature into functional cells.” (1)
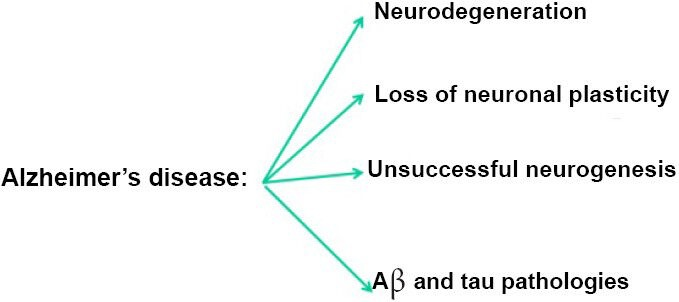
“Alzheimer’s disease is characterized by neurodegeneration associated with loss of neuronal plasticity and in the dentate gyrus, proliferation of newborn cells which do not mature into functional neurons…” (1)
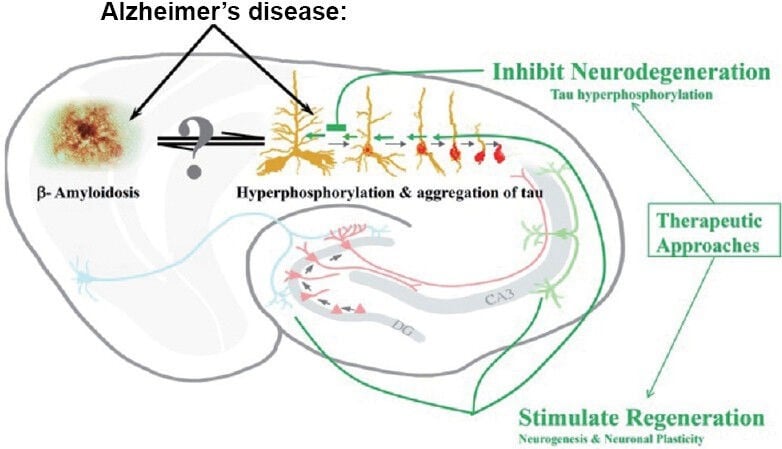
“Two major therapeutic approaches to Alzheimer’s disease and related conditions.
While one therapeutic approach to Alzheimer’s disease is the inhibition of neurodegeneration that is associated with neurofibrillary and Aβ pathologies, another approach is to stimulate the regeneration of the brain by enhancing neuronal plasticity and neurogenesis that culminates into formation of mature functional neurons.” (1)
Proper neurogenesis (birth of new neurons) by peptide P021 was shown to remove Tau protein aggregates and reduce the production of new Beta Amyloid plaques.
“Moreover, the P021 treatment markedly reduced tau pathology and attenuated the generation but not the clearance of Aβ in 3xTg-AD mice.” (2)
“Cognitive performance was studied by assessing episodic memory with Novel Object Recognition task at 16-17-months post-treatment. We found that P021 treatment initiated during the synaptic compensation period can prevent neurodegeneration, Aβ and tau pathologies, rescue episodic memory impairment, and markedly reduce mortality rate. These findings for the first time show effective prevention of AD changes with a neurotrophic compound that targets neurogenesis and synaptic plasticity, suggesting that improving the health of the neuronal network can prevent AD.” (3)
“The AD brain responds to neurodegeneration by stimulating neurogenesis, however, because of the lack of a proper neurotrophic microenvironment of the hippocampus, this effort of the AD brain to replace lost neurons with new neurons is unsuccessful and culminates in failure of neuronal survival, maturation, and integration. As the disease progresses, the neurogenic failure becomes severe, and contributes significantly to cognitive decline.” (4)
Mechanisms of Peptide P21 (P021):
“We summarize the preclinical studies with a ciliary neurotrophic factor (CNTF) small-molecule peptide mimetic, Peptide 021 (P021). P021 is a neurogenic and neurotrophic compound which enhances dentate gyrus neurogenesis and memory processes via inhibiting leukemia inhibitory factor (LIF) signaling pathway and increasing brain-derived neurotrophic factor (BDNF) expression. It robustly inhibits tau abnormal hyperphosphorylation via increased BDNF mediated decrease in glycogen synthase kinase-3β (GSK-3β, major tau kinase) activity. P021 is a small molecular weight, BBB permeable compound with suitable pharmacokinetics for oral administration, and without adverse effects associated with native CNTF or BDNF molecule. P021 has shown beneficial therapeutic effect in several preclinical studies and has emerged as a highly promising compound for AD drug development.” (4)
“The IL-6 family of cytokines includes CNTF, interleukin-11 (IL-11), leukemia inhibitory factor (LIF), oncostatin-M, cardiotrophin-1, and cardiotrophin-like cytokine. CNTF plays a pivotal role in adult hippocampal and subventricular zone neurogenesis, and the differentiation of neural stem cells. CNTF signaling occurs through a tripartite complex of CNTF receptor α (CNTFRα), LIF β receptor (LIFR), and glycoprotein 130 (gp130). CNTF and LIF both signal through tyrosine phosphorylation (Tyr706) of the signal transducers and activators of transcription (STAT) proteins by the membrane associated Janus kinase (JAK). In the brain, CNTF is expressed in astrocytes in the neurogenic niches, while its receptor, CNTFRα, is expressed predominantly in neural progenitor cells and hippocampal neurons, and various other areas of the brain including motor cortex and cerebellum. Overall, CNTF is the most extensively studied neuropoetic cytokine, and its neuroprotective effects are well established. CNTF administration has been shown to alleviate cognitive impairment and to stabilize synaptic protein levels in a transgenic mouse model of AD.” (4)
“A major problem associated with the therapeutic usage of whole molecule neurotrophic factors is the pleiotropic actions that derive from their concomitant binding to multiple receptors. The small-molecule mimetics might also allow to modulate various aspects of these signaling pathways in ways that are distinct from the whole molecule neurotrophic factors. By departing from conventional neurotrophic factor signaling, these small-molecule mimetics might provide a novel therapeutic approach to treat AD.” (4)
“Oral administration of the compound P021 could rescue cognitive aging by enhancing neurogenesis via increased BDNF expression and by decreasing tau levels in aged Fisher rats.” (4)


“The effect of P021 on tau and probably also on Aβ pathology was via increased BDNF expression mediated activation of TrkB-PI3K-AKT signaling pathway which leads to down-stream inhibition of GSK-3β activity by increase in its inhibitory phosphorylation at Ser9 by AKT. GSK3β is a major tau serine/threonine kinase which is known to phosphorylate tau at many different sites including Ser199, Ser202, Thr205, Ser396, and Ser404. Reduction in GSK3β activity has also been demonstrated to ameliorate Aβ pathology via reduction in amyloidogenic processing of APP.” (4)
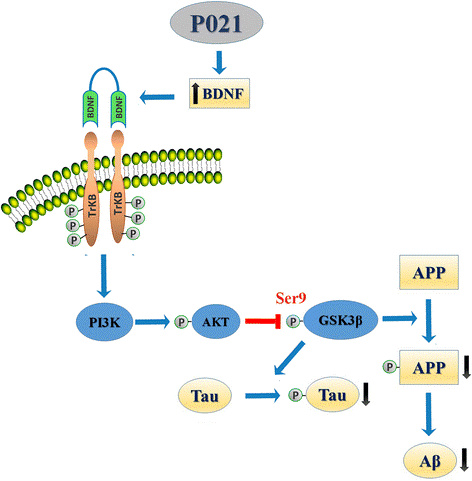
- “P021 attenuated robustly tau and mildly Aβ pathologies in 3xTg-AD mice.
- Disease-modifying effect of P021 was probably via BDNF/TrkB/PI3-K/AKT/GSK3β pathway.
- P021 could rescue deficits in neurogenesis, neuronal plasticity, and cognition.
- P021 is a potential disease-modifying treatment for Alzheimer’s disease.” (5)
“Here we investigated the effect of chronic oral treatment with a ciliary neurotrophic factor (CNTF) derived peptidergic compound, P021, on disease pathology both at moderate and severe stages in a transgenic mouse model of AD. 3xTg-AD and wild type female mice were treated for 12 months with P021 or vehicle diet starting at 9–10 months of age. A significant reduction in abnormal hyperphosphorylation and accumulation of tau at known major AD neurofibrillary pathology associated sites was observed. The effect of P021 on Aβ pathology was limited to a significant decrease in soluble Aβ levels and a trend towards reduction in Aβ plaque load in CA1 region of hippocampus, consistent with reduction in Aβ generation and not clearance. This disease modifying effect was probably via increased brain derived neurotrophic factor (BDNF) expression mediated decrease in glycogen synthase kinase-3-β (GSK3β) activity we found in P021 treated 3xTg-AD mice. P021 treatment also rescued deficits in cognition, neurogenesis, and synaptic plasticity in 3xTg-AD mice. These findings demonstrate the potential of the neurotrophic peptide mimetic as a disease modifying therapy for AD.” (5)
Sourced Studies:
Product available for research use only:

